Optimizing the Parameters for Microwave UV Curing
by Madeline Kraizel and Hallie Smith-Petee
Nordson Corporation
UV curing can be a fairly simple process spanning many industries. A coating is applied to a part and passed under a UV lamp, which cures the coating almost instantaneously. UV curing is used on many products, from cell phones and car headlights to magazine covers and medical devices (See Figure 1).
There are a number of reasons UV curing is so appealing. The extremely fast cure time means it is a perfect candidate for high speed production; this, in turn, leads to a small space requirement. Since the cure time is almost instantaneous, parts can pass under a UV lamp system without impeding production rates, while large convection ovens take up much more space, time and energy. The smaller, more compact line promotes lean manufacturing. The characteristic fast cure time also results in a decreased risk of contamination. In addition to the short cure time, UV also offers a low-temperature solution to cure parts (like some plastics) that may not hold up to the temperatures of a convection or infrared (IR) oven.
Removing contaminants from the air supply and near the UV curing area is critical as contaminants in the air can settle on bulbs and reflectors in the UV system. Dirt or other contamination will inhibit the UV output of the bulb. Reflectors inside the UV lamp can be adversely affected by dirty air. A dirty reflector will not perform optimally and will affect the quality of the cure. Filters should be used to clean the air circulation around the UV system. A preventative maintenance schedule also should be established to periodically clean bulbs and reflectors to maximize performance and life of these items.
Contamination also can be generated by a UV system that uses aluminum components. Aluminum will react during the UV process to create aluminum oxide; a chalky substance that can become airborne during the curing process and land on parts. This contamination will cause surface defects, especially noticeable on Class A surfaces. The aluminum oxide build-up will shorten the life of an aluminum reflector. Over time, the corrosive nature of aluminum oxide will result in the replacement of these reflectors, which can be costly and cause unnecessary down time.
The amount of energy transferred to a part, also called energy density, is a critical factor of UV curing. Energy density is the volume of UV that the surface sees over a period of time. Energy is cumulative, so the desired energy density can be achieved by adjusting production line speed or utilizing multiple lamps. In most cases, if a customer wishes to run faster production rates, multiple lamps may be required to achieve the proper amount of energy to fully cure the material. The material suppliers can help determine the amount of energy required for the coating to cure. It is very important to understand the energy requirements to optimize the UV curing system. If the proper amount of energy density is not achieved, the cure will not be completed, resulting in underdevelopment of coating properties. Depending on the coating, the result may be poor weathering performance or a decrease in scratch resistance.
Intensity or irradiance, the power or brightness of the lamp, is an important parameter for proper curing of UV coatings. Intensity is expressed in watts per square centimeter or milliwatts per square centimeter. The peak irradiance required for curing must be considered when establishing a UV system. The irradiance is not cumulative over multiple lamps, so the system must have proper equipment to reach the power needed to cure effectively.
In addition, choosing a bulb with the proper spectrum and intensity is important to successfully cure a part. There are a wide variety of UV coatings and photoinitiators, and there are six different electrodeless bulbs that may be selected. UV light, just like visible light, is a spectrum, and there are different energies that are associated with each wavelength of UV light. These energies penetrate and cure each coating differently, based on the wavelength used. The most common bulb used is a mercury (H) bulb. This gives a broadband output across all wavelengths, and some versions of the mercury bulb have enhanced performance in the UVC range. Iron (D) doped bulbs work well for UVA output, while gallium (V) bulbs work well for parts of the UVV range. Gallium bulbs operate up to 420nm, while Indium (Q) bulbs work well in the UVV range up to 450 nm. The right bulb must be chosen based on the photoinitiator used, coating thickness and cure properties.
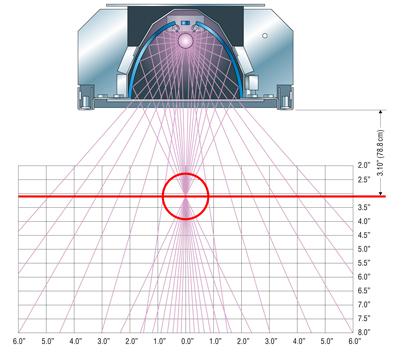
Choosing the proper reflectors for the UV system will enhance the ability to cure parts more efficiently. Reflectors are available in three different focal point options: 2.1", 3.1" and flood. The focal point is the distance of the part from the lamphead or irradiator to the most intense area of UV light. A 3.1" focal point (See Figure 2) reflects all the UV rays to a distance of 3.1" from the lamphead. Another benefit of using a 3.1" reflector is a lower temperature exposure to the substrate because it is farther away from the heat of the bulb. This area provides the highest intensity of UV light.
Three dimensional parts also can be cured using UV light. The important thing to remember is that all areas of the part must be exposed to the light to cure. Multiple lamp systems can be configured to surround the part with UV. Secondary reflectors (not "regular" mirrors, they do not reflect UV rays) also can be incorporated to ensure that the light reaches all areas of the part.
Another critical parameter of the UV system is the amount of oxygen or ozone. All UV curing processes create ozone, a corrosive gas. Typically, the ozone is removed using a vent or an open air system. In some cases, the amount of oxygen can inhibit the curing process. As mentioned earlier, UV coatings cure by photoinitiators being excited by UV light, resulting in atoms being cleaved. These atoms then bond with other monomers and oligomers to form a highly cross-linked, durable coating. Free radical oxygen is very reactive and will bond with many things. Oxygen will bond with monomers, oligomers and intermediate chains, but oxygen also can bond directly to the photoinitiators. No matter what the oxygen bonds to, it essentially stops the cross-linking process needed to cure the coating. Long, cross-linked chains cannot, and will not, be formed. This leads to coatings that lose their properties, such as durability, hard finish, glossy finish and scratch/heat resistance. Signs of oxygen inhibition include a tacky or sticky finish, drop in coating performance, longer cure times and a decrease in coating thickness.
Some solutions may include adding excess photoinitiator to the coating to bind with the excess oxygen to complete the reaction. Too many photoinitiators, however, can result in coatings that yellow and have an odor. Increasing the intensity of the UV lamp is another possible solution. But, the most effective way to avoid oxygen inhibition is to remove the oxygen. This can be done by performing the curing process in an inert environment. Nitrogen or carbon dioxide can be used to reduce the amount of oxygen in the air. Reducing the oxygen from about 210,000 parts per million to below 50 parts per million is ideal in preventing oxygen inhibition. By effectively eliminating the oxygen in the air, oxygen inhibition can be avoided. New curing systems have been designed to provide a nitrogen-enriched chamber for the parts to cure (See Figure 3).
The almost instantaneous cure time, paired with other benefits, such as being environmentally friendly, make UV curing an attractive substitute to conventional convection ovens. While oxygen inhibition may be a potential reason to avoid UV cures, new technology provides easy solutions. UV curing still can produce the hard, durable coating expected without the risk of oxygen inhibition by utilizing nitrogen inert atmospheres. New conveyor systems can be equipped with nitrogen chambers to combat oxygen inhibition.
Recognizing the critical parameters of microwave UV systems ensures that high-quality parts are created at the end of the process. Users may want to consider implementing a preventative maintenance plan to reduce potential contamination and change appropriate spare parts periodically. These simple activities will allow users to achieve a consistent, worry-free system to cure any part. All in all, UV curing is the optimal process to produce hard, scratch-resistant, durable and long-lasting coatings on a large variety of products.